Home> News
Researchers discover new mechanism of metformin-activated CMA for treating AD pathologies
Updated: 2021-12-02
Alzheimer's disease (AD), also widely known as senile dementia, is the most common neurodegenerative disorder. Patients with senile dementia are mainly plagued by memory loss and loss of the ability to care for themselves.
In addition, they suffer from dysthymic disorder and loss of the ability to move around independently. The disease has a significant impact on patients’ personal and family lives, as well as social development in general. Statistics show that the number of AD patients has surpassed 50 million worldwide.
It is expected that the number will rise to 152 million by 2050. The amount of money spent globally on medical treatment and rehabilitation for patients with senile dementia stands at $1 trillion and will roughly double in 2030.
Over 100 AD drug candidates have undergone clinical trials, but only seven of them have gained market approval since 1998. International pharmaceutical giants have suffered setbacks to varying degrees in their attempts to produce effective AD drugs over the years.
For example, there some doubts about the efficacy of Aduhelm, a new Alzheimer's drug developed by US pharmaceutical company Biogen, casting a shadow over mankind's dream of curing the disease.
Along with her fellow researchers, Ana Maria Cuervo, a professor at the Albert Einstein College of Medicine in the US, recently found that the activation of chaperone-mediated autophagy (CMA) can reverse key symptoms associated with senile dementia in mice.
Relevant findings from their laboratory research were published in their study "Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome" in the journal Cell on April 22.
Although their research on the use of activated CMA to treat AD has brought a ray of hope to patients with senile dementia, there are no related drug candidates in clinical trials at the moment.
CMA is a selective autophagy that can facilitate the degradation of proteins. The efficiency of CMA tends to decrease as people age.
This phenomenon increases the risk for substrate proteins to form insoluble blocks that are detrimental to human cells. In fact, the aggregation of toxic proteins in human brains is what AD and many other neurodegenerative diseases have in common.
A study group headed by Professor Xia Hongguang at the School of Basic Medical Sciences and Liangzhu Laboratory of Zhejiang University published their research findings, titled "Metformin activates chaperone-mediated autophagy and improves disease pathologies in an Alzheimer disease mouse model", in the journal Protein & Cell on July 21.
During their research, the employment of high throughput screening methods in the selection of drugs suggested that metformin is a novel CMA activator for diseases. Researchers discovered the first reported mechanism for CMA regulation via the metformin-TAK1-IKKα/β-Hsc70 signaling pathway and found that the Aβ amyloid-beta precursor protein (APP) is a new CMA substrate.
Their findings indicated that introducing metformin or over-expressing Hsc70 to the hippocampuses of laboratory mice could activate CMA and dramatically alleviate AD pathologies. The research identified metformin as a new drug to treat AD in clinical trials and uncovered a new mechanism of metformin-induced activation to treat AD.
Xia’s group has been committed to the research of selective CMA in the regulation of human diseases. Xia and his teammates published a research paper, titled "Pharmacological targeting of MCL-1 promotes mitophagy and improves disease pathologies in an Alzheimer's disease mouse model", in the journal Nature Communications on Nov 12, 2020.
They found that UMI-77, a molecular compound, can also alleviate AD pathologies by inducing the autophagy of mitochondria.
Two separate innovative works have unanimously confirmed that the selective degradation of toxic aggregated proteins and broken mitochondria is a promising approach to treat AD and other neurodegenerative diseases.
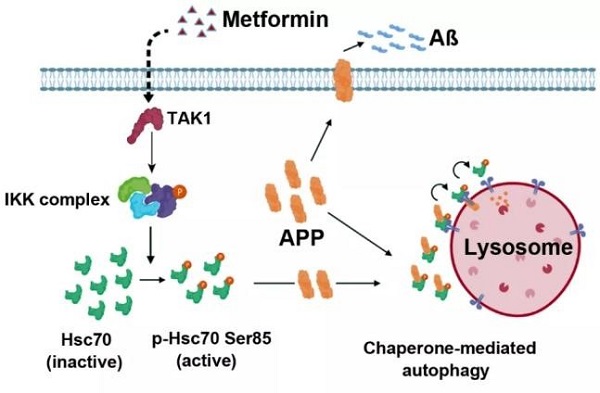
Metformin can induce CMA activation and facilitate the degradation of APP substrate proteins.
Xu Xiaoyan and Cen Xufeng, postdoctoral researchers at the School of Basic Medical Sciences and Liangzhu Laboratory of Zhejiang University, Sun Yaqin, a doctoral student at the same school and laboratory in Zhejiang University, and Shan Bing, a research fellow at the Shanghai Institute of Organic Chemistry in the Chinese Academy of Sciences, were the first authors of the publication.
Xia is its major corresponding author. Professor Ayaz Najafov is another corresponding author of the paper.
